Background : Multiple myeloma (MM) is a subclonal disease characterized by large heterogeneity with a complex treatment landscape. Patients with MM have a myriad of available treatment options, prescribed based on several factors, including genetic testing, comorbidities, risk status, age, patient preference, and/or prior treatment failures. Cytogenetic abnormalities determines risk stratification and prognosis. The prognostic significance of t(11;14)-positivity has been debated and it is currently considered a standard-risk abnormality; however, testing for t(11;14) may be of potential therapeutic significance. Despite recent advances in treatment that have improved response rates and survival, MM remains incurable. This results in a continuous treat-to-failure approach that eventually leads to a lack of effective therapeutic options. Treatment patterns and outcomes for patients by lines of therapy (LOT) in real-world settings provide important benchmarks for assessments of future targeted therapies.
Methods : Data were retrospectively collected from the ConcertAI Patient360 database containing aggregated electronic health record data of patients treated in primarily community-based oncology centers in the US. Adults diagnosed with MM (2011-2022) who received a National Comprehensive Cancer Network-guideline recommended treatment and had ≥2-months follow up were included; this analysis focused on patients with relapsed or refractory (RR) MM who are t(11;14)-positive and received ≥2 prior lines of systemic therapy. Baseline characteristics were determined for the third-line plus (3L+) subgroup of patients with t(11;14) RRMM. Real-world progression-free survival (PFS) and overall survival (OS) were estimated by the Kaplan-Meier method. Results were stratified by the use of doublet or triplet regimens, in which patients were treated with 2 or 3 classes of treatments among 6 options (ie, proteasome inhibitors [PI], immunomodulatory imide drugs [IMID], anti-CD38 monoclonal antibodies, chemotherapy, BCL-2 inhibitors, exportin-1 inhibitors, or steroid therapy), respectively, at any time during the LOT.
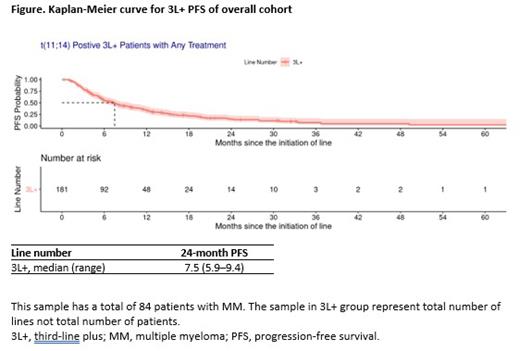
Results : Eighty-four patients with t(11;14) RRMM received 3L+ of therapy. Of patients, 43% were female, 20% were Black, and 75% were White. The median (range) age at diagnosis was 64 (37-84), and the median (range) follow-up since diagnosis was 76 (10-209) months. At 24 months since the initiation of treatment, the median (range) PFS for all t(11;14)-positive patients that received 3L+ of therapy was 7.5 (5.9-9.4) months, and the median (range) PFS rate was 14.8% (9.7%-22.5%) (Figure); the median (range) OS for all t(11;14)-positive patients that received 3L+ of therapy was 32.1 (25.4-48.0) months, and the median (range) OS rate was 58.3% (51.0%-66.6%). The most common treatment regimens used in 3L+ included Daratumumab + Pomalidomide ± Dexamethasone (12%), Venetoclax monotherapy (9%), Pomalidomide ± Dexamethasone (7%), and Bortezomib ± Dexamethasone (7%). Of doublet regimens used in 3L+ (n = 69 LOT), 18 (26%) were IMID + steroid, 14 (20%) were PI + steroid, and 11 (16%) monoclonal antibody (MA)+ steroid. For triplet regimens used at 3L+ (n = 97 LOT), 28 (29%) were IMID + MA + steroid, 23 (24%) were IMID + PI + steroid, and 12 (12%) were MA + PI + steroid. Triplet regimen use increased from 52% in 3L to 61% in 4L and was associated with slightly longer PFS in 3L, relative to doublet regimen use. For patients that received 3L+ therapies, the median (95% CI) PFS was 6.7 (5.4-11.1) months for patients receiving doublet regimens, and 7.7 (5.9-11.3) months for patients receiving triplet regimens.
Conclusions: Triplet regimens were more commonly used than doublet regimens as 3L+ therapy. Triplets were also associated with slightly longer PFS in 3L; however, PFS rates were low and did not translate into longer OS, suggesting an unmet need for novel therapeutic options for patients with t(11;14) RRMM.
Disclosures
Girvan:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Martins:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Yu:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Ng:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Kamalakar:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Zafar:Genentech/Roche: Current Employment, Current holder of stock options in a privately-held company. Tulsian:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Cornell:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company. Samir:AbbVie Inc: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal